Which of the Following Temperatures Is the Lowest
0663 580 times 10-4 580 0581 172 Round the number 0007222 to three significant figures. Which of the following is are the lowest temperature.

The Lowest Temperature At Which An Oil Gives Sufficient Vapours To Form An Explosive Physics Questions Temperatures Vapor
Above what temperature does the following reaction become nonspontaneous feo given the following equation n2og no2g 3nog.

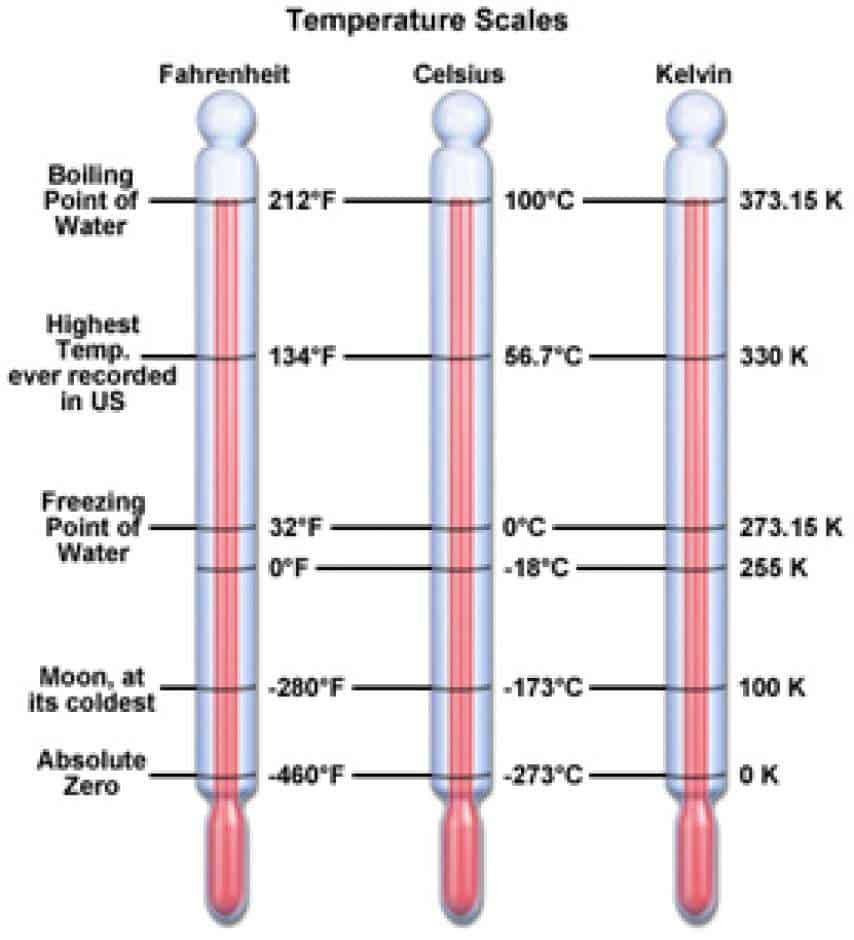
. Fahrenheit Temperature Celsius Temperaturex95 32. Place the highest temperature on the left side of the screen and the lowest temperature on the right side of the screen. 100C is 2376F and 373K.
100K is -173C and -2538F. 100 degrees Celsius is the highest temperature. MgBr 2 at 25C d.
Which of the following represents the lowest temperature that can be theorectically achieved. Transcribed image text. A 313 K C 75F B 48C D All of these temperatures are all equal.
Which of the following does not characterize at least some low-latitude climates. At low temperatures ΔG will be negative because of the effect of the negative ΔH but as you increase the temperature the effect of the positive -TΔS will eventually outweigh that. 100F is 378 C and 3107K.
High temperatures on clear days. What volume in cm3 would be occupied by a 333 g sample of gold. C The solute-solvent attractions are very strong.
D The solute is insoluble in the solvent. Rank the following temperatures from highest to lowest. There are also temperature scales in which zero is absolute zero the lowest possible temperature.
C 3H 7OH at 75C c. Which of the following climate types experiences wet winters and dry summers. B The solute-solute attractions are much stronger than the solvent-solvent attractions.
Which of the following does not exert a major influence on low-latitude climates. For an exothermic solution which of the following statements is true. A The solute will be more soluble in the solvent at high temperatures.
Chemistry questions and answers. C 5H 11OH at 75C 18. The freezing point of water 5 degree C 30 degree F 280 K A and D Gold has a density of 001932 kgcm3.
There are two hydrogen bonds between one adenine and thymine base pairs where as there are three hydrogen bonds between one guanine and cytosine base pairs. Which of the following climate types experiences low precipitation all year and is dominated by cT air masses. Which of the following is not a low-latitude climate region.
5 degrees C 30 degrees F 280 K A D. People have gotten close to absolute zero but have never reached it. QUESTION 2 Which of the following is the lowest temperature.
According to theory we never will. So the DNA with maximum number of A-T pairing will melt faster at lower. A 75F B 348 K C 72C D All of these temperatures are all equal.
Questions in other subjects. C 5H 11OH at 25C e. The energy required to break the bond between G-C will be more as compared to energy required for A-T bonds.
C 3H 7OH at 25C b. How many joules of heat are absorbed to raise the temperature of 125 grams of water at 1 atm from 35C to its boiling point 100C. Which of the following is the lowest temp.
Predict which of the following liquidtemperature scenarios would have the HIGHEST vapor pressure and the. 9 Which of the following is the lowest temperature. Which of the following liquids would have the lowest viscosity factoring in both the impact of the substance and the temperature.
15 Which of the following is the lowest temperature. 參75F O 72C O 348 K Al of these temperatures are all equal.

No comments for "Which of the Following Temperatures Is the Lowest"
Post a Comment